[Legal Updates] Guidelines for Chemical & Biological Drugs
2025. 2. 26
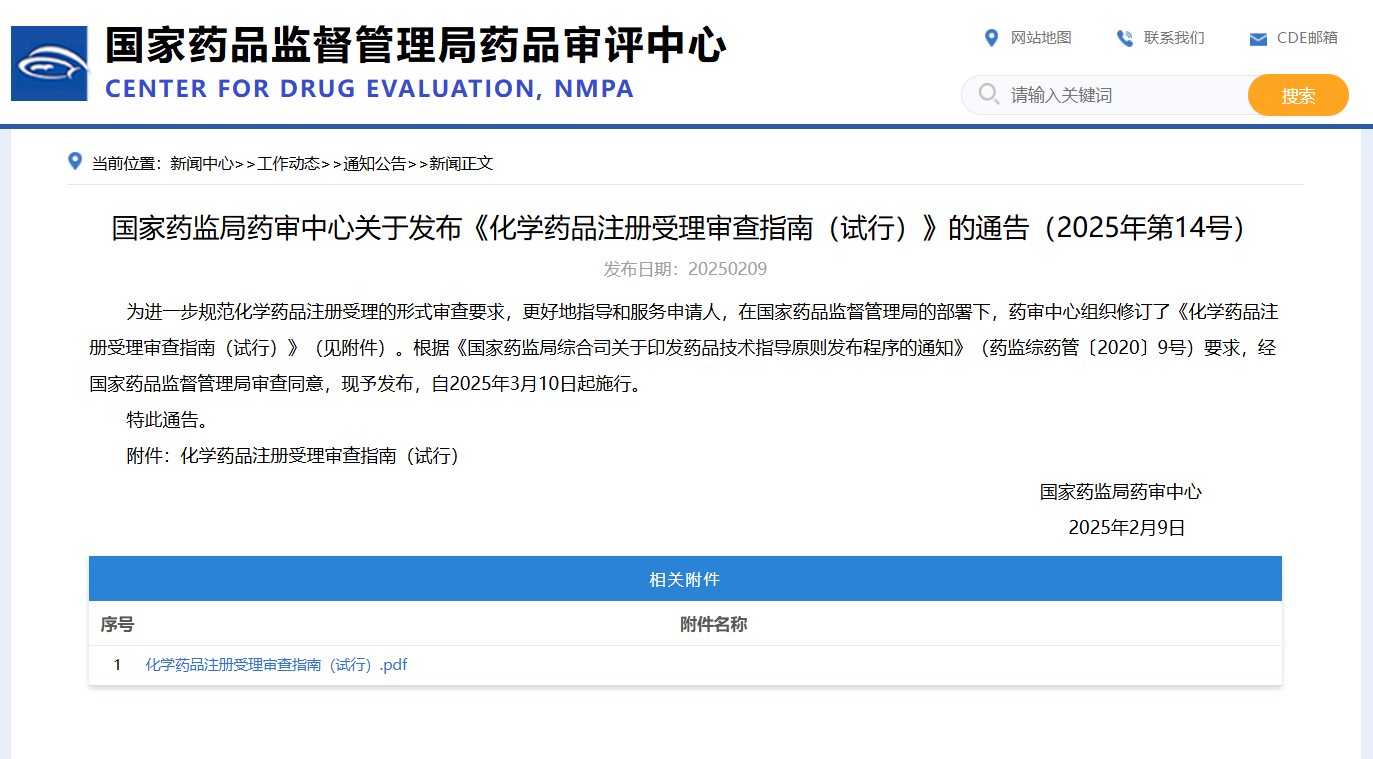
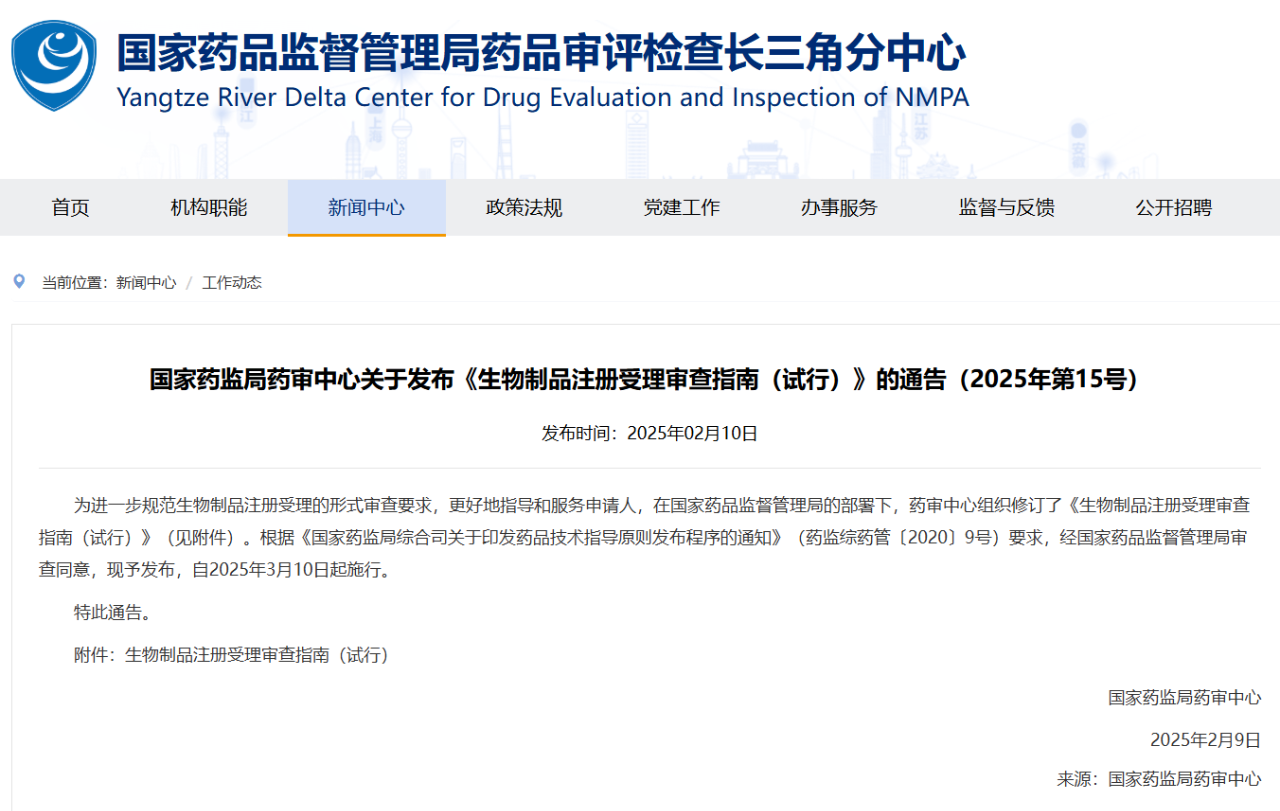
The Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) has recently released the Guidelines for the Acceptance and Review of Chemical Drug Registration (Trial) and the Guidelines for the Acceptance and Review of Biological Product Registration (Trial). These two documents provide comprehensive guidance for the registration applications of chemical drugs and biological products, clearly outlining the acceptance and review procedures, the required documentation, key review points, and the criteria for acceptance decisions.
Compared to the 2020 version, this revision introduces several enhancements, including more detailed formal review standards, strengthened electronic application procedures, and reinforced patent compliance management. These measures aim to further refine the registration review process, improve its transparency and efficiency, and ensure the safety and quality of pharmaceutical products.
Sources: